DEPTH (Dual Enumerative Point-of-care Test for HIV) is a microfluidic assay that is designed to be cheap, portable, and accurate. It is perfect for clinical testing of HIV in third-world nations, where access to more expensive machinery is unavailable.
Background
There are design concerns to HIV diagnostics in third world nations due to cost limitations, leaving designers with dichotomous options:
Quantitative vs. Qualitative
Mobile vs. Immobile
Cheap (<$5 per test) vs. Expensive (>$5 per test)
Ideally, researchers want to develop a diagnostic that can be used clinically in third world nations. This diagnostic must be mobile and cheap, and ideally can quantify the progression of the disease.
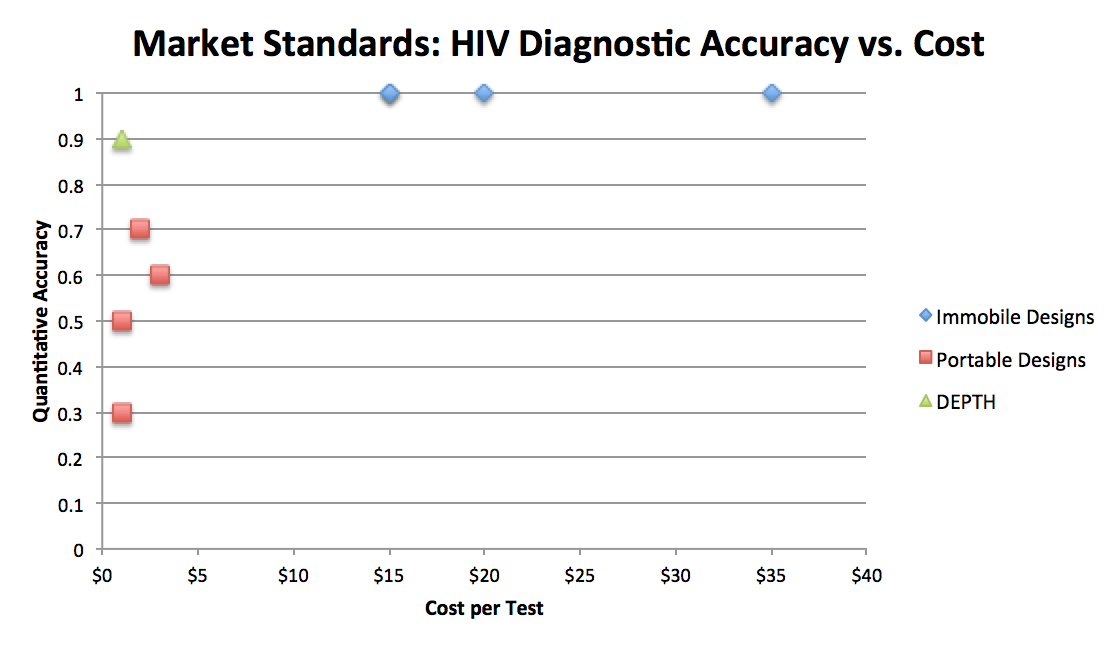
Market Research
Current diagnostics on the market fall into two categories: they are either expensive and accurate, or cheap and get the job done. The extent of HIV infection is most often characterized by a patient's CD4+ T Cell count. As HIV progresses into AIDS, a patient's CD4+ cell count drops as their immune system weakens. Expensive tests such as Nucleic Acid Amplification, Flow-Assisted Cell Sorting, or Western Blots cost at least $15 per test, and are not financially viable in African nations where HIV is most prevalent. Cheap tests are either qualitative or semi-quantitative.
Quantitative designs such as Visitect's HIV exam, which functions similarly to a pregnancy stick, or Zyomyx's design, which functions similarly to a mercury thermometer, currently dominate the market. However, Visitect's design only characterizes whether the patient has hit a CD4+ cell density threshold (350 cells/µl), and Zyomyx's design has notable inaccuracy due to the random nature of space filling.
Qualitative designs simply determine whether there is a viral load present in the sample. These designs do not track the progression of the disease, but they give immediate results (whereas CD4+ cell counts generally remain unaffected until approximately 1 month following infection). SD Bioline suffers from a non-intuitive design, so users must be literate in order to use the device. Capillus designed another microfluidic viral load chip with decent accuracy, which functions using a latex that agglutinates when exposed to HIV antigens. The Capillus HIV test is used by the World Health Organization and can provide nice, immediate results.
To try to get the best of both worlds, my team developed and prototyped a microfluidic assay which we named DEPTH (a Dual Enumerative Point-of-care Test for HIV). DEPTH would function with a cell-phone app, where we would image the chip and quantify the level of fluorescence to provide a quantitative and accurate metric for HIV progress.
The Design
We had a few goals with our design. We wanted our design to be scientifically accurate, produce reliable results, and most importantly we needed a design that was user-friendly. Reading could be done a few ways: using agglutination (qualitative), indicator markings (tiered), or by fluorescent imaging (spectral). We chose to use colorimetric fluorescence because it allows the user to identify a spectrum of potential disease progression. One of the primary concerns with colorimetric diagnostic tools is that users will often either (i) misread the device or (ii) purposely misinterpret the device if it has bad news. In order to avoid potential patient denial, we decided it would be best to integrate the chip with cellular imaging to control for that variable.
This process would be straight-forward, had we gone to manufacturing. Fluorescent imaging must be done in darkness - to accomplish this, simply use the box in which the test would be shipped as a "dark room" by designing the box with a small hole on the top to allow the cell phone to sit and image. Literary research regarding cellular resolution, reliability, and antibody quantification showed no immediately foreseeable issues with this design.
We created DEPTH using AutoCAD, and then manufactured a physical prototype via PDMS microfluidic fabrication.
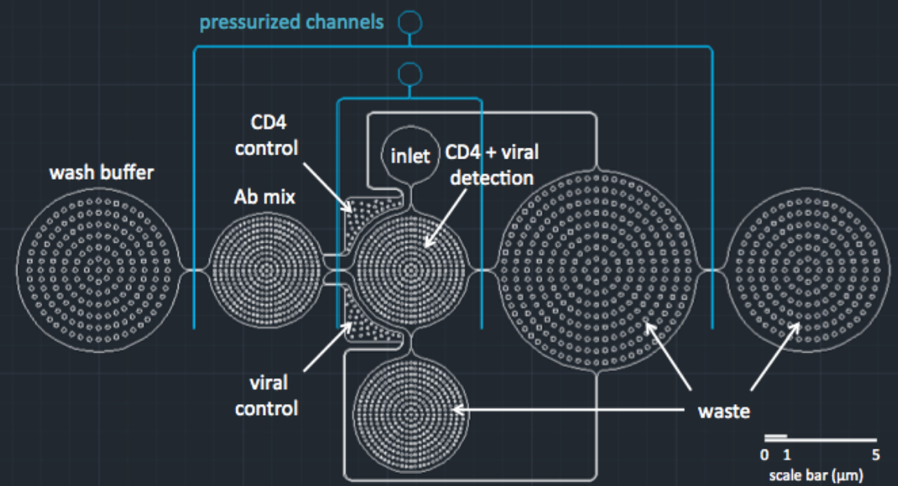
DEPTH
Prototype Testing
Flow testing: we first had to show that blood could be injected into the inlet and would perfuse through the device (red). Then, the wash (blue) should wash the blood away, leaving behind only CD4+ cells that have been caught by their antibodies. This allows for accurate enumeration of cells without any materials that would block visibility.
Modeling: using COMSOL, we developed a simplified verison of our cell capture chapter to analyze what kinds of flow rates and capture rates we would need in order to get reliable data.
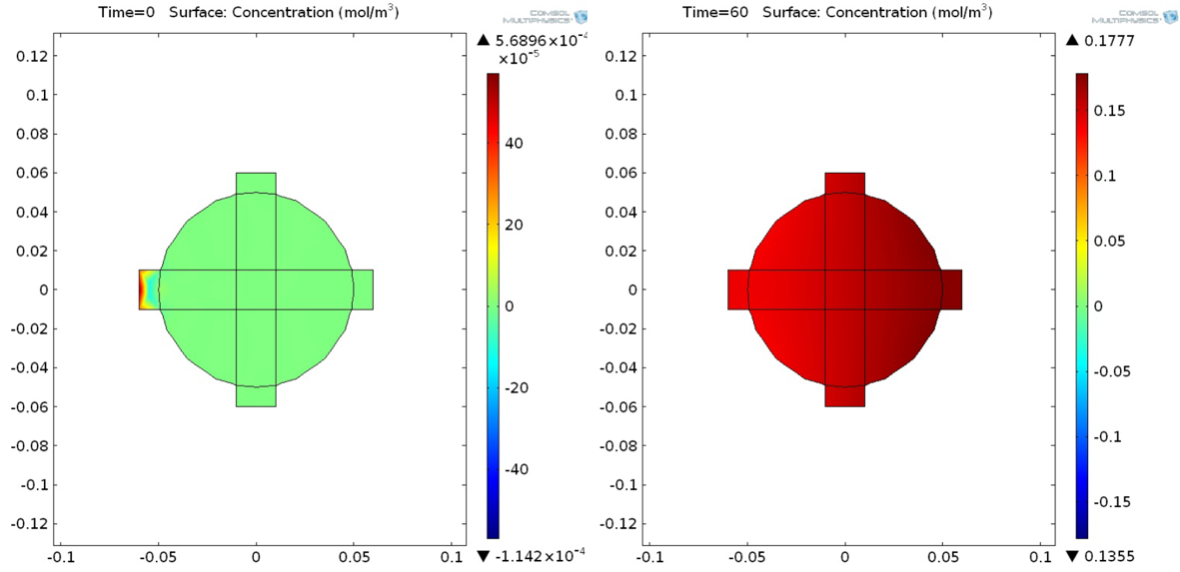
[Other testing not shown: microbead fabrication, magnetic capture efficiency]
New Design Considerations
Our secondary design, POCAHONTAS, which was developed but not prototyped, included user improvements over the first. Both designs were made to work with punch valves (convenient compared to traditional methods, because no external equipment is needed to release the valve). However, POCAHONTAS only consisted of a single valve, meaning that users would only have to interact with the device once after the initial sample was loaded. Given the dangerous nature of potentially HIV positive biological samples, it is important to limit physical interaction with the device. A single valve is easier to use, safer, and we were able to set it pretty far away from the rest of the microfluidic, meaning a lower chance of clinicians being at risk of infection.
We also designed POCAHONTAS to contain more control regions, which ensures more reliable data. We changed the geometry of our device in two ways: we created a smaller detection chamber (which ensures more equal distribution of our sample, and a smaller capture region for the camera) and we stratified our channels to ensure that our wash buffer would equally pass over the entire fluid regions. These changes are primarily to emphasize clean engineering and more reliable data.
Conclusions
As an early prototype, our device worked on all experimental fronts and showed potential to function quickly and reliably in a clinical setting! Microfluidics are considered by many to be the premier path for diagnostics both in afflicted societies and in first world nations. This proof of concept represents a new method to quantify HIV rapidly, which could help organize treatment for millions of affected patients.
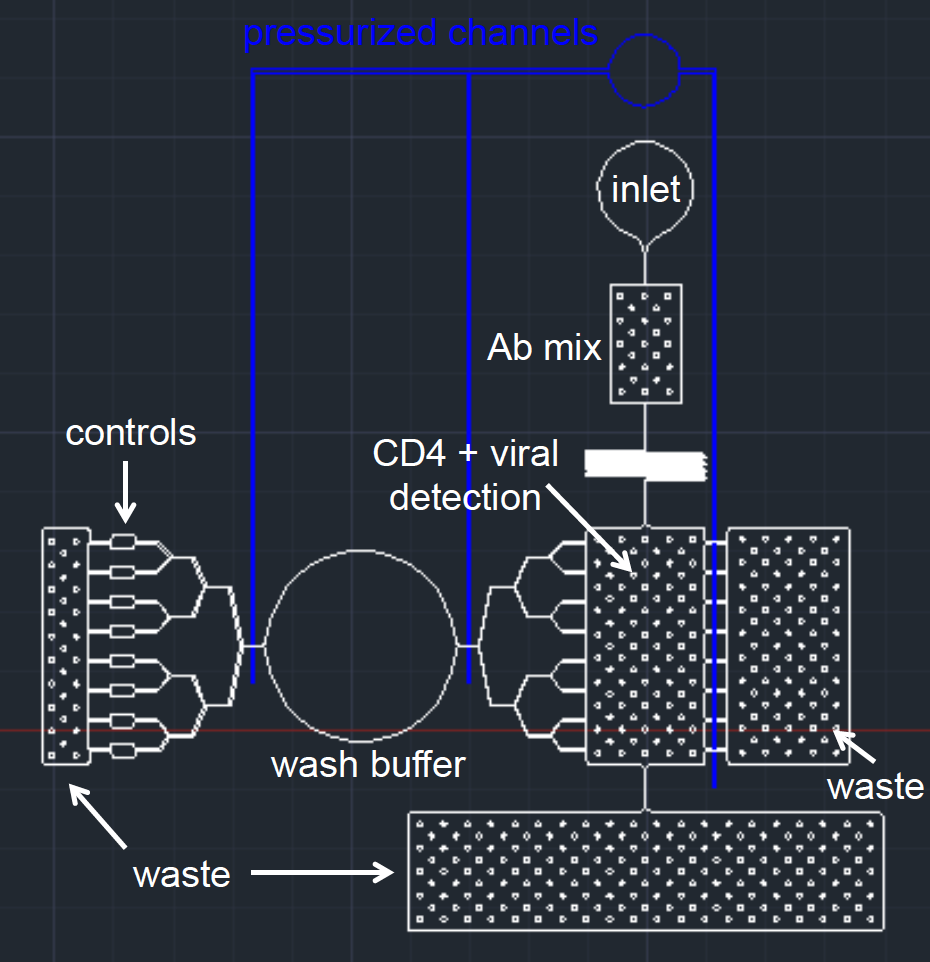
POCAHONTAS