CITO is a new platform for liquid biopsy analysis of cell free DNA (cfDNA) to monitor response to anti-cancer treatment.

CITO provides the crucial information needed by physicians to understand the effectiveness of a patients treatment plan allowing continuation or rapid alteration. To do this, whole blood is processed and cfDNA is isolated to determine the presence of specific sequence mutations using digital PCR.

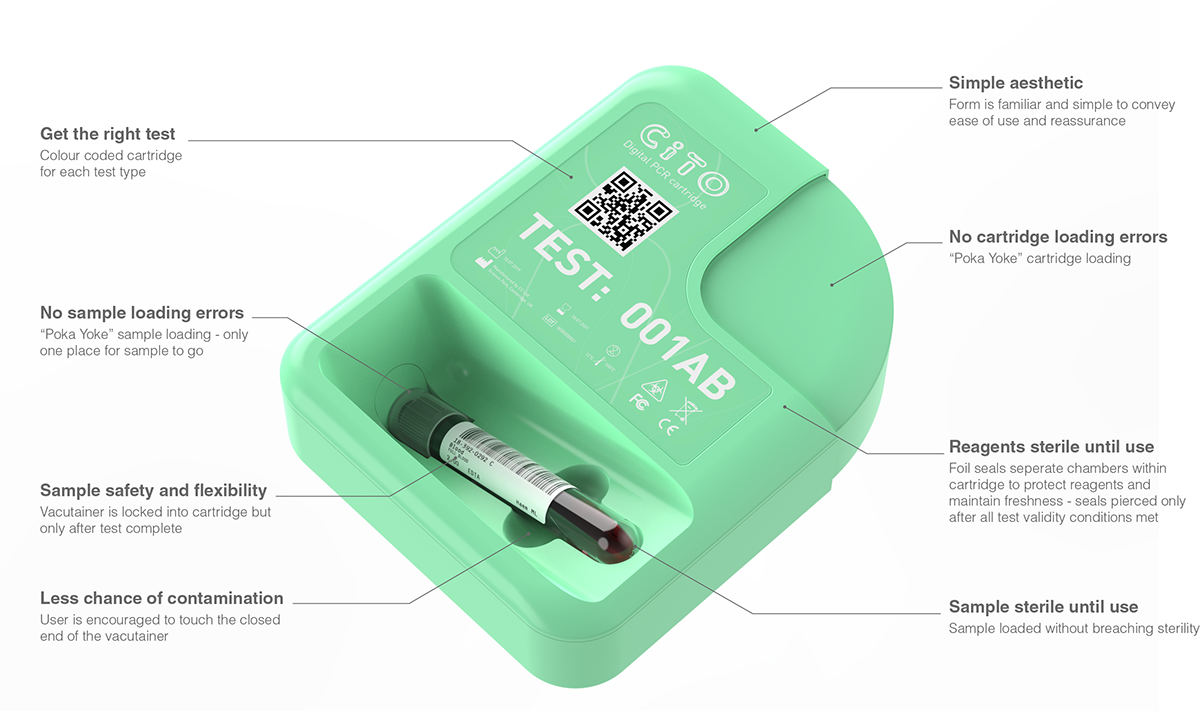
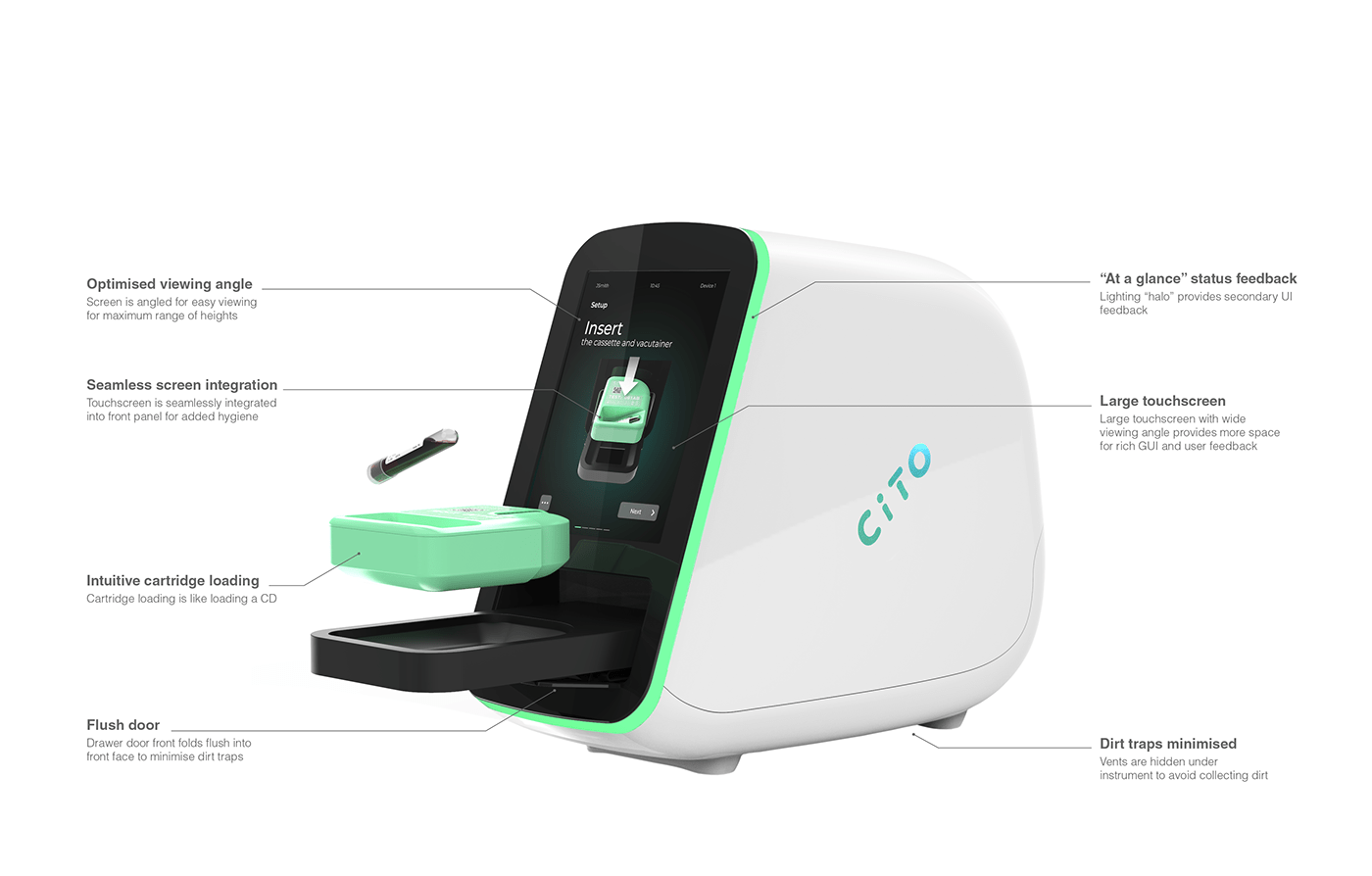
The CITO system consists of a test specific single-use cartridge and diagnostic instrument.

The area of cancer diagnostics is complex and there are many challenges to successfully developing and launching a new product into this market. The cancer patient care pathway involves many people, places and technology. Our team broke this down to its constituent parts to understand where key pain points and areas of opportunity were.
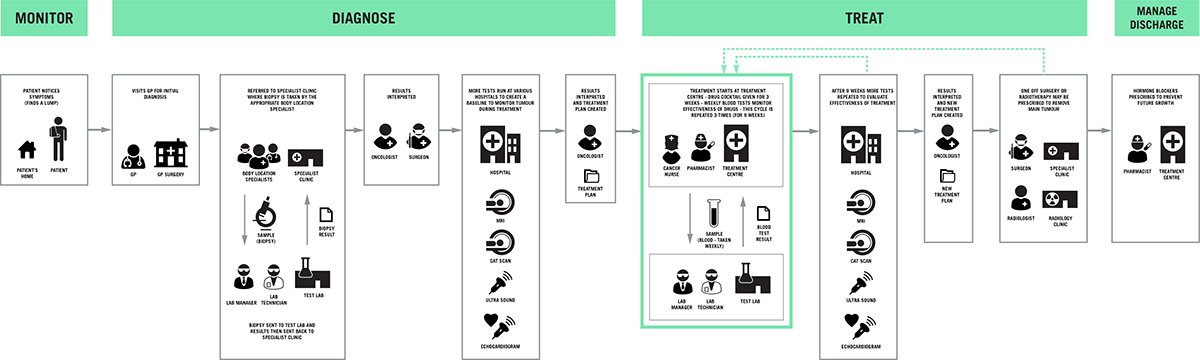
This identified treatment monitoring as an area where rapid diagnostics could identify the effectiveness of treatment plans.
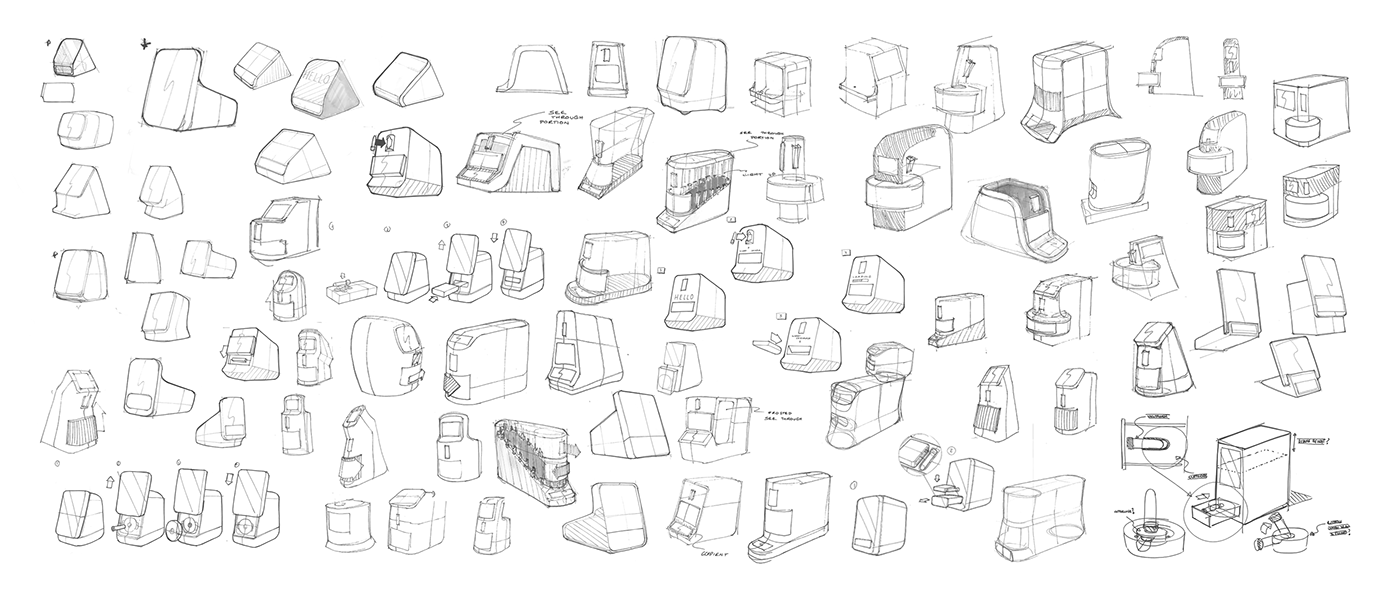
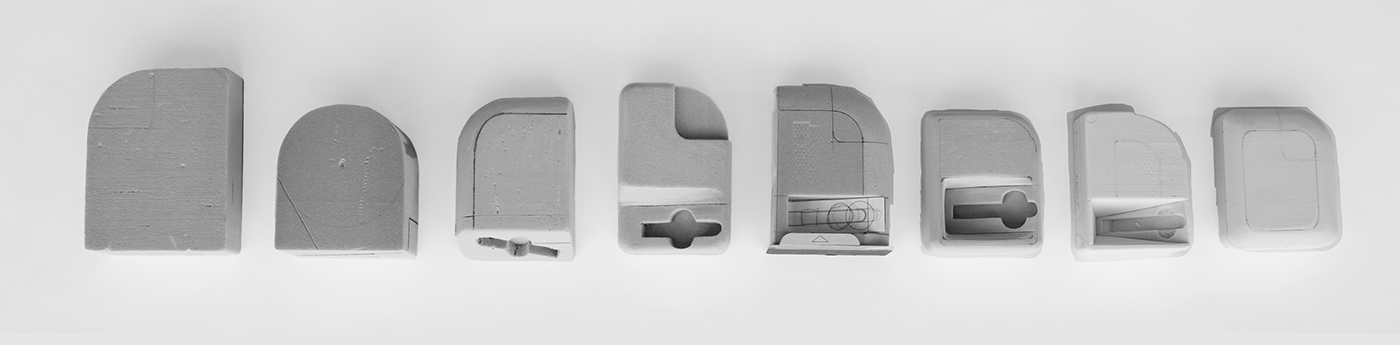
Form and functionality of instrument and cartridge were explored through 2D sketching then 3D block modelling.

Development of the brand identity started with exploration of the unique nature of an individual cell, whilst also considering key system attributes such as effectiveness and efficiency.
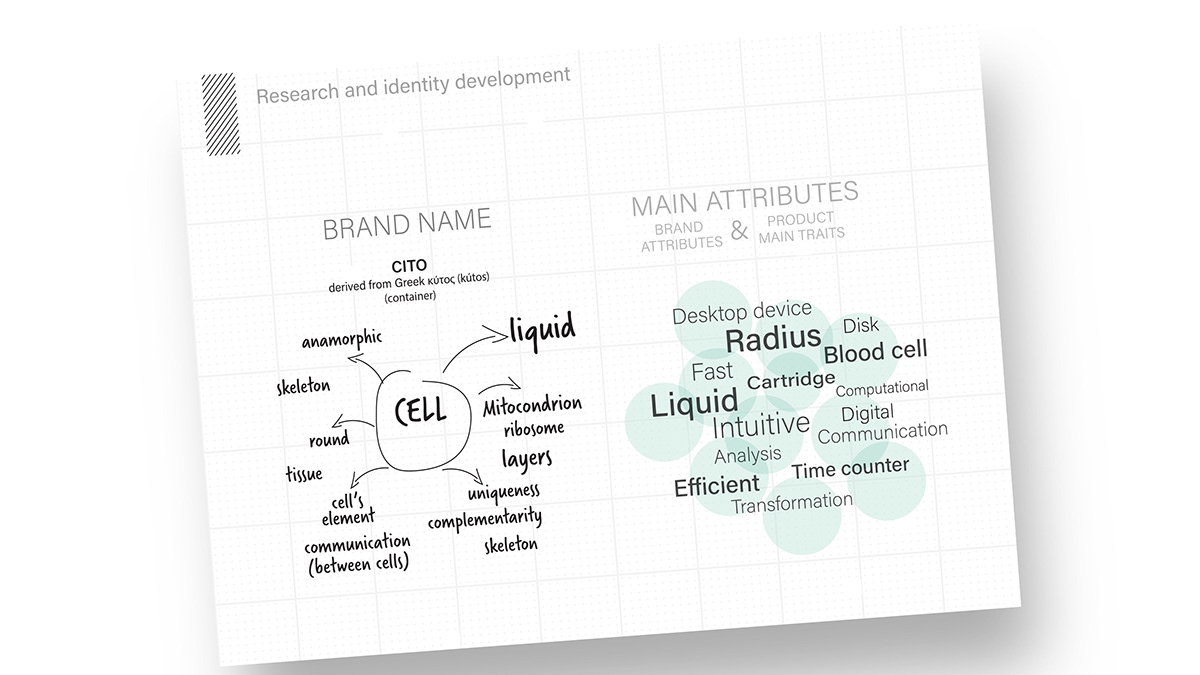
The logotype portrays the main characteristics of the cell, referencing the cell's structural components and becomes a typographical play around the concept of modularity, movement and uniqueness.


The primary users of the CITO system are low skilled lab workers who don't diagnose results themselves. These users and the environment in which CITO will be used demands that the solution must be simple and intuitive to use, minimise the potential for errors, be space efficient and importantly, be easy to clean and prevent contamination.

The instrument scans the user's face and unlocks if the user is authorised to continue

A 10ml sample of whole blood is loaded, via a vacutainer, into the CITO cartridge which contains all the reagents needed to perform the test. The sample and cartridge are loaded into the instrument via the drawer. On-screen animations guide the user through the process.

The instrument verifies that the sample and test being performed are correct. The sample, which is extremely valuable, remains sterile and can be removed and reused up until the point that the test has been initiated by the user.

Test progress feedback is displayed on-screen with "at a glance" feedback provided via the halo ring.

On successful completion of a test, data is automatically sent to the physician. The user also has the option to review the results locally if needed.