MyLabBuddy provides mathematical and physical science support to life science researchers. Researchers can calculate the concentration of a solution in molarity, percent weight or percent volume, they can read protocols, and formulate buffers. MyLabBuddy is implemented in Java with an attractive Java Swing GUI and intuitive controls.

MyLabBuddy - theme art by OCAL from www.clker.com/clipart-flask-icon.html.

Class Dependency Graph for MyLabBuddy

Make a solution by molarity. Enter the formula weight, compound name and final volume.

Make a percent solution by weight. Concentration can be specified by weight or by volume.
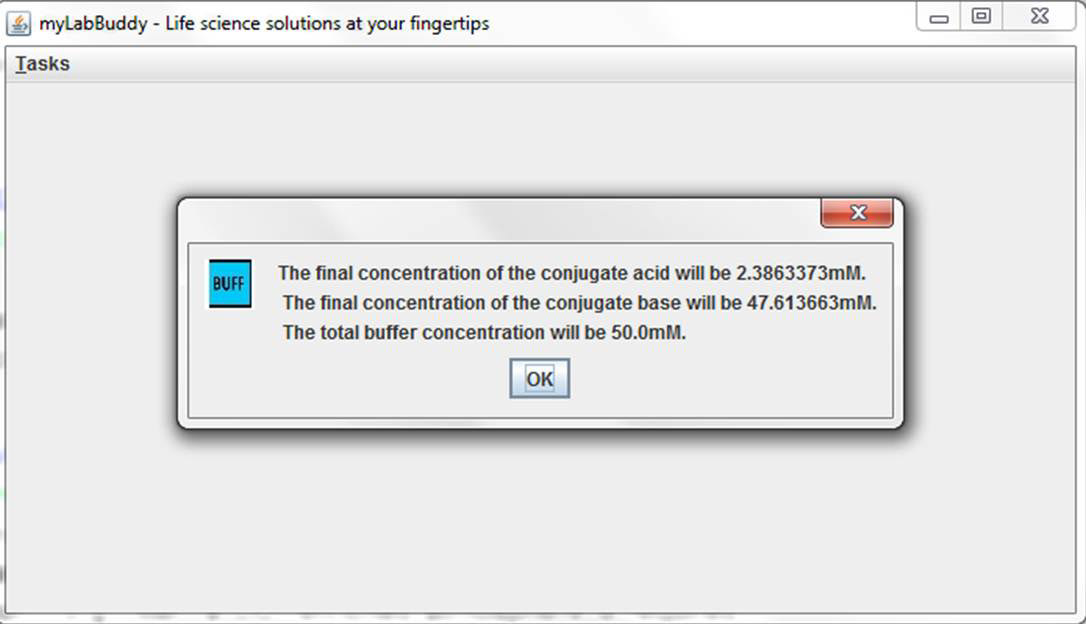
Formulate a buffer. Enter the pKa of the conjugate acid, the desired pH and the total concentration of the conjugate acid and conjugate base. Bicarbonate buffer has a pH of 6.1 and is commonly used to make pH 7.4 buffer. Bicarbonate buffer is the buffer system used in the blood. Rapid breathing (hyperventilation) removes carbon dioxide from the blood increasing the pH of the blood (by removing carbonic acid from the circulation) producing a state known as respiratory alkalosis.

Display a protocol. Here the protocol for making a solution is displayed.

All icons are royalty-free and the flask icons are modifications of the original flask (top) by OCAL at www.clker.com/clipart-flask-icon.html. The lower two (square) icons were designed by me.