International Year of Chemistry
2011 print series
2011 print series
At the end of 2009 you might remember that I designed the prints for the International Year of Astronomy which, unexpectedly, was a massive hit and really helped promote a worthwhile campaign. Now we are in 2011 and this year it is the turn of the International Year of Chemistry.
The prints below have all been inspired by a famous chemist or discovery. For full details of these and if you want to purchase one then see my portfolio site at excite.co.uk.
The Designs

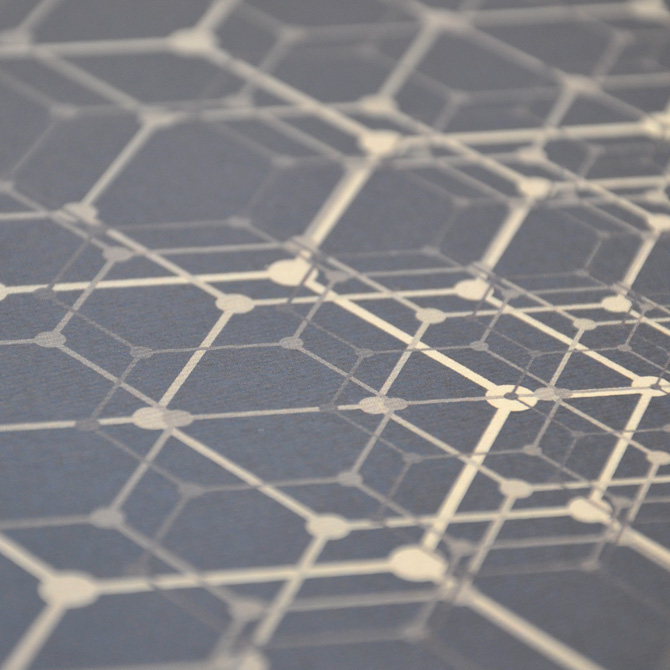
Atomise (John Dalton)
John Dalton (6 September 1766 – 27 July 1844) was an English chemist and physicist; professor of mathematics and natural philosophy (1793); developed atomic theory; his theory (1805) accounts for the law of conservation of mass, law of definite proportions and law of multiple proportions; produced the first table of atomic weights; colour-blind and mostly self-taught.

Ions (Lorenzo Avogadro)
Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto (9 August 1776, Turin, Piedmont – 9 July 1856) was an Italian savant. He is most noted for his contributions to molecular theory, including what is known as Avogadro's law. In tribute to him, the number of elementary entities (atoms, molecules, ions or other particles) in 1 mole of a substance, 6.02214179(30)×10^23, is known as the Avogadro constant.
Available in Blue (Limited) or Red
Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto (9 August 1776, Turin, Piedmont – 9 July 1856) was an Italian savant. He is most noted for his contributions to molecular theory, including what is known as Avogadro's law. In tribute to him, the number of elementary entities (atoms, molecules, ions or other particles) in 1 mole of a substance, 6.02214179(30)×10^23, is known as the Avogadro constant.
Available in Blue (Limited) or Red

Matter (Albert Einstein)
Albert Einstein (14 March 1879 – 18 April 1955) was a German theoretical physicist and chemist. Einstein's most famous discovery was the theory of general relativity. Einstein’s 3rd paper on Brownian Motion confirmed the atomic theory of matter. This is viewed by many as the first proof that atoms actually exist.

Atom (Ernest Rutherford)
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS (30 August 1871 – 19 October 1937) was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics. In early work he discovered the concept of radioactive half-life, proved that radioactivity involved the transmutation of one chemical element to another, and also differentiated and named alpha and beta radiation, proving that the former was essentially helium ions. This work was done at McGill University in Canada. It is the basis for the Nobel Prize in Chemistry he was awarded in 1908 for his investigations into the disintegration of the elements, and the chemistry of radioactive substances materials.

Striking (Buckyballs)
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are called buckyballs, and resemble the balls used in football. The first fullerene to be discovered was prepared in 1985 by Richard Smalley, Robert Curl, James Heath, Sean O'Brien, and Harold Kroto at Rice University. The name was an homage to Buckminster Fuller, whose geodesic domes it resembles. According to astronomer Letizia Stanghellini, "It’s possible that buckyballs from outer space provided seeds for life on Earth.

Revolution (Graphene)
Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer carbon foils in 1962. Graphene has been touted as the "miracle material" of the 21st Century. Said to be the strongest material ever measured, an improvement upon and a replacement for silicon and the most conductive material known to man.

H20 (Henry Cavendish)
Henry Cavendish FRS (10 October 1731 – 24 February 1810) was a British scientist noted for his discovery of hydrogen or what he called "inflammable air". He described the density of inflammable air, which formed water on combustion, in a 1766 paper "On Factitious Airs".

Emission (Marie Curie)
Marie Skłodowska Curie (7 November 1867 – 4 July 1934) was a Polish-born French physicist and chemist famous for her pioneering work on radioactivity. She was the first person honored with two Nobel Prizes — in physics and chemistry. The contributions that Marie Curie made to science are immense. With her help, doctors have been able to treat cancer, manipulate nuclear energy and numerous other achievements have stemmed from her work.
This design was initially created for a screening of a Marie Curie documentary.
This design was initially created for a screening of a Marie Curie documentary.

Substance (Antoine-Laurent Lavoisier)
Antoine-Laurent Lavoisier was born in 1743. He studied mathematics and astronomy with Nicolas de Lacaille (1713-1762), chemistry with Guillaume-François Rouelle (1703-1770) and botany with Bernard de Jussieu (1699-1777) at the Collège Mazarin. From 1763-1767 he studied geology under Jean Etienne Guettard (1715-1786). He was one of the best known French scientists and an important government official. His theories of combustion, and his development of a new system of chemical nomenclature and the first modern textbook of chemistry led to his being known as the father of modern chemistry. As a scientist, Lavoisier demonstrated the nature of combustion, disproving the phlogiston theory. He also proposed the name "oxygen" for the substance previously known as "dephilogisticated air," and laid the framework for understanding chemical reactions as combinations of elements to form new materials.

Elements (Dmitri Mendeleev)
Dmitri Ivanovich Mendeleev (8 February 1834 – 2 February 1907) was a Russian chemist and inventor. He made a number of important contributions but is famously credited as being the creator of the first version of the periodic table of elements. Using the table, he predicted the properties of elements yet to be discovered. Element number 101, the radioactive mendelevium, was later named after him.
The Prints



Details
Prints are available and ship internationally see link below. They are printed on museum-quality paper which is internally buffered to resist fading and acid-free to eliminate any degradation. The inks have a very high pigment density, which allows for the sharpest possible image, perfect reflection of light and the widest range of printable colour. The pigment also resists water, light, and gas for superior archiving properties. All prints have a minimum 1" white border. Available in the following sizes: 13" x 19" / 18" x 24" / 24" x 36" (limited).
> Purchase prints
n.b. For those in the UK, 13" x 19" signed prints on 250gsm Tintoretto Gesso with a 1" border are available directly, contact me for more details.
n.b. For those in the UK, 13" x 19" signed prints on 250gsm Tintoretto Gesso with a 1" border are available directly, contact me for more details.

