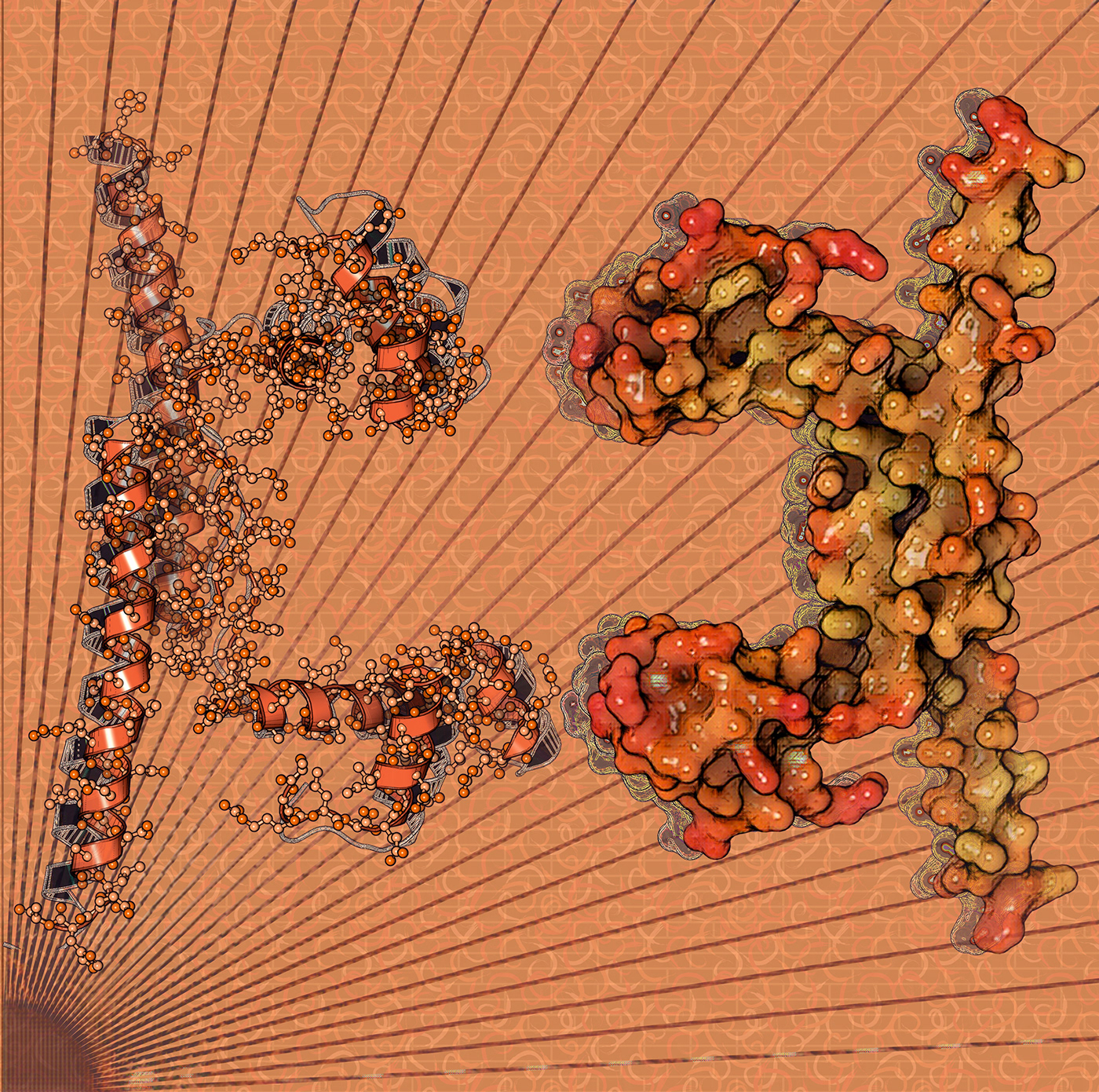


DNA-binding proteins include transcription factors which modulate the process of transcription, various polymerases, nucleases which cleave DNA molecules, and histones which are involved in chromosome packaging and transcription in the cell nucleus. DNA-binding proteins can incorporate such domains as the zinc finger, the helix-turn-helix, and the leucine zipper (among many others) that facilitate binding to nucleic acid. There are also more unusual examples such as transcription activator like effectors. Structural proteins that bind DNA are well-understood examples of non-specific DNA-protein interactions. Protein–DNA interactions occur when a protein binds a molecule of DNA, often to regulate the biological function of DNA, usually the expression of a gene. Among the proteins that bind to DNA are transcription factors that activate or repress gene expression by binding to DNA motifs and histones that form part of the structure of DNA and bind to it less specifically. Also proteins that repair DNA such as uracil-DNA glycosylase interact closely with it. In general, proteins bind to DNA in the major groove; however, there are exceptions. Protein–DNA interaction are of mainly two types, either specific interaction, or non-specific interaction. Recent single-molecule experiments showed that DNA binding proteins undergo of rapid rebinding in order to bind in correct orientation for recognizing the target site. Here you can see the crystal structure of the apo-unbound form of ArdK, a DNA-binding protein encoded within the R388 plasmid from Escherichia coli (PDB code: 7BBQ)
#immolecular ...#molecularart ... #DNA ... #binding ... #plasmid ... #escherichia ... #xray
Structure rendered with @proteinimaging and composed by @corelphotopaint
#immolecular ...#molecularart ... #DNA ... #binding ... #plasmid ... #escherichia ... #xray
Structure rendered with @proteinimaging and composed by @corelphotopaint

